In the leaching residue of the platinum- rich nickel- copper copper leached with concentrated hydrochloric acid to remove nickel, copper is mainly present in the form of copper sulfide. The liquid chlorination method leaches copper sulfide by introducing chlorine into a mixed slurry of a copper-containing, nickel-containing hydrochloric acid solution and copper sulfide-leaching slag. In order to prevent the precipitation of cuprous chloride during the leaching process, the leachate must contain a chloride such as nickel chloride or free hydrochloric acid. At this point, the chlorination of copper is:
2Cu + +Cl 2 ![]() 2Cu 2 + +2Cl - (1)
2Cu 2 + +2Cl - (1)
Cu 2 S+Cu 2 + ![]() CuS+2Cu + (2)
CuS+2Cu + (2)
CuS+Cu 2 + ![]() 2Cu 2 + +S (3)
2Cu 2 + +S (3)
S+2e ![]() S 2 - (4)
S 2 - (4)
Cu 2 + +S 2 - ![]() CuS (5)
CuS (5)
The complete leaching of copper depends on the reaction formula (3). The reaction formulas (4) and (5) only indicate whether copper is precipitated as sulfide or by adjusting the redox potential of the leaching process (measured by platinum and saturated calomel electrode insertion solution) to bring copper into solution? That is, at a high oxidation-reduction potential, the reaction proceeds according to the formula (3); and at a low oxidation-reduction potential and a specific temperature, acidity, and copper concentration, the progress of the reaction formulas (4) and (5) is accelerated. A large amount of sulfur ions and sulfides are generated. When the concentration of copper sulfide exceeds its solubility product, a copper sulfide precipitate is formed, at which time the copper cannot be completely leached.
The minimum redox potential necessary for copper to leach as completely as possible depends primarily on the copper concentration, acidity and temperature in the solution. However, in practice, the potential range of the leaching operation (Fig. 1) is between 0.35 and 0.45V. Within this range the highest potential leaching of copper oxide, and the noble metal is substantially insoluble. This may be because the precious metal does not dissolve in this potential interval, or may react with copper. After dissolution, the reaction is re-formed by the reaction of (6) and (7):
S+ 2 e ![]() S 2 - (6)
S 2 - (6)
P 3 + +S 2 - ![]() PS (7)
PS (7)

Figure 1 Dissolution rate of different potentials
During the leaching process, all free selenium will react with precious metal ions (possibly like formulas (6) and (7)) to form insoluble selenide precipitates.
In order to increase the leaching rate of copper and to prevent precious metals from entering the solution as much as possible, a suitable redox potential can be selected from the curve of Fig. 1 in advance. It should be noted, however, that the dissolution profiles of copper and precious metals in the graph are affected by changes in copper concentration, acidity, and temperature in the solution. When operating at high acid, low copper concentrations and high temperature operating conditions, the curve shifts slightly to the left; at low acidity and high copper concentration and low temperature operating conditions, the curve shifts slightly to the right.
The process of leaching platinum-rich nickel-copper copper by aqueous solution chlorination is also suitable for treating the original high-ice nickel containing the precious metal, sulfur and selenium in the plant. When the high ice nickel leaching slag composed of the above composition is leached at the selected oxidation-reduction potential, the leaching slag is leached by tetrachloroethylene to remove sulfur, and the content of the precious metal in the concentrate is 100 times higher than that of the high ice nickel. Therefore, the method can treat both the platinum-rich nickel matte and the high-ice nickel to recover the precious metal concentrate. This will reduce the factory's delivery of intermediate ice high nickel to the Eagle Bridge plant and take advantage of the nickel refining capabilities of the Norwegian plant.
Since thick film heater element is new type fluid heater parts for most home appliances manufacturers or other customers, we make this Smart temperature control testing kit, which can be set to supply target temperature hot water or water volume in each single heating process.
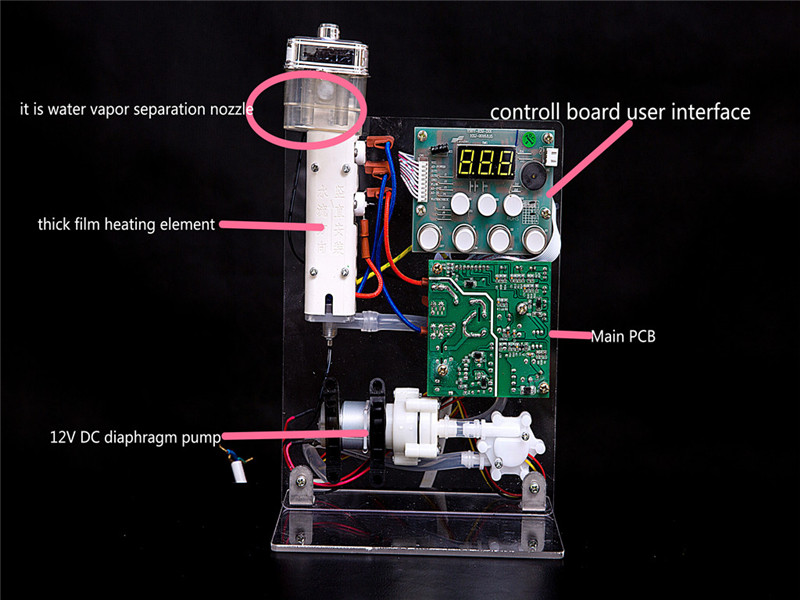
Smart temperature control testing kit is assembled by Thick Film Heater Element, DC Membrane Pump,
PCB control system, LCD Display, Touch Buttons etc.
Instant Heating Test Kit,Instant Heating Test ,Electrical Instant Water Heating ,Heat Loss Testing Machine
XINXIANG JIEDA PRECISION ELECTRONICS CO.,LTD , https://www.gidaheater.com